Condensed Summary here.
Part 1 Chemistry Extended Summary:
Sn1 Reactions; Step 1-Leaving Group Departure
An important type of reaction in organic chemistry is a nucleophilic substitution reaction.
A nucleophilic substitution reaction occurs when a nucleophile (either a single atom or several atoms
bonded together) is attracted to a positive charge or a nucleus. A nucleophile is usually a negatively
charged group, and in a nucleophilic substitution reaction, it is replaced by another. A nucleophile
can be a single atom such as a halide (like chlorine or iodine), denoted by X, or a molecule with a
negative charge (alcohol functional groups (OH) or a cyanide group (CN)). Some nucleophiles are
more electronegative, and therefore, better nucleophiles than others.
There are two types of nucleophilic substitution reactions: unimolecular and bimolecular,
refered to as Sn1 and Sn2, respectively. In this case, we are talking about an Sn1 reaction, which takes
place in several steps. The word Sn1 comes from the fact that the rate determining step for an Sn1
reaction consists of only one substance; an Sn1 reaction consists of only one nucleophile interacting
with the central carbon atom at all times.
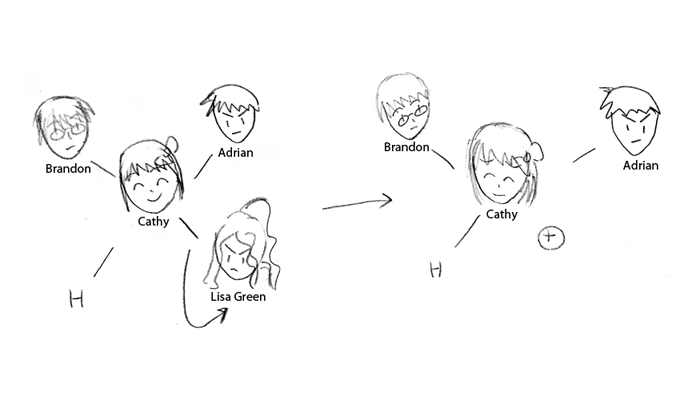
In an Sn1 reaction, the first step is when the nucleophile leaves. In this case, the nucleophile
is called a leaving group (LG). A leaving group will usually leave because it is stable enough on its
own. Leaving can be assisted through the use of heat and certain solvents—generally a leaving group
will leave when in a protic solvent that contains free protons that can help stabilize the leaving group.
Additionally, an Sn1 reaction only occurs when the carbon that the leaving group is attached to will
still be stable enough even after the leaving group leaves. The molecule that forms from the departure
of the leaving group is a positively charged carbon, or a carbocation; while still not completely stable,
it is stable enough to exist temporarily in solution.
In the case of this story, Lisa Green is the leaving group—such as a halide—in the molecule.
The leaving group has high electronegativity, pulling electrons away from the central atom and closer
to itself; Lisa's selfishness leads to her pulling out of the group. Lisa is able to leave due to the fact that
she is stable enough on her own-she's a strong enough singer-that she can handle being on her own. Her
leaving places a great deal of pressure on Cathy, the central carbon atom; Cathy becomes positively
charged, a carbocation, and is very strained in the environment.