Part 1 Chemistry Summary:
Sn1 Reactions; Step 1-Leaving Group Departure
The first part illustrates the departure of a leaving group in an Sn1 reaction. In this story, Lisa Green is the leaving group in the molecule. A leaving group has high electronegativity, pulling electrons away from the central atom and closer to itself; Lisa's selfishness leads to her pulling out of the group. Lisa is able to leave due to the fact that she is stable enough on her own-she's a strong enough singer-that she can handle being on her own. Likewise, only relativity stable substituants will leave, taking a pair of electrons with them. Her leaving places a great deal of pressure on Cathy, the central carbon atom; Cathy becomes positively charged, a carbocation, and is very strained in the environment.
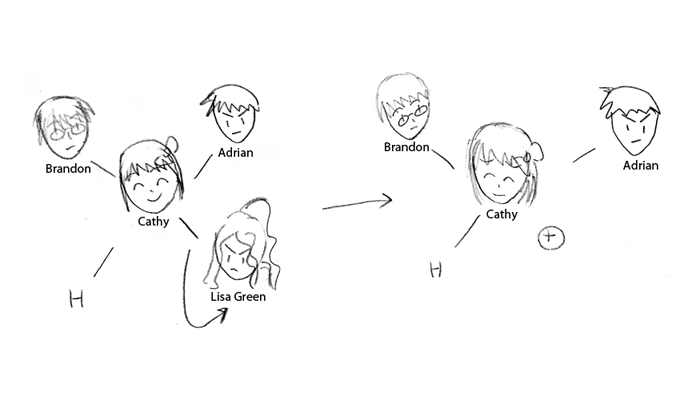
Extended Summary here.

