Concept Explaination:
Catalyzed Reactions:
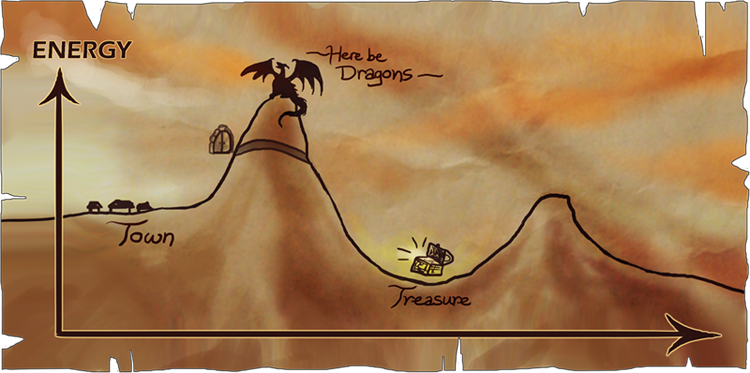
This story illustrates the energetics of a chemical reaction. Chemical reactions are endothermic or exothermic: endothermic reactions result in a higher energy product than the starting material, consuming heat; and exothermic reactions result in a lower energy product, releasing heat. Reactions require activation energy, often supplied by heat. In this story, the adventurers attempt to undergo an exothermic reaction. They stockpile on energy, eating a great deal of food in preparation for the dragon, which represents the activation energy at the peak of the mountain. They hope that by defeating the dragon, they can gain treasure, which will give them a great deal of energy—an exothermic reaction—so they can live a more peaceful life.
However, some reactions cannot go to completion without a catalyst. A catalyst is a reagent that changes the rate of a reaction by binding with the reactants to change the reaction pathway. Essentially, catalysts open a new path. When the adventurers reach the peak, they do not have enough energy to defeat the dragon, because the activation energy is too high! Luckily they meet a man, who joins them briefly to show them a secret passageway through the mountain, revealing the catalytic path that is lower in energy. As a result, they easily transverse the activation energy and end up reaping the energetic reward of a lower net energy.